The RF Compliance coding system allows pharmaceutical manufacturers to comply the FMD (Falsified Medicines Directive).
With the ability to generate, print and verify serialised 2D codes on cartons, the RF Compliance offers an instant, reliable and easy-to-use solution for pharmaceutical manufacturers who need to meet the 2019 FMD deadline. As an offline (or “near line”) system, the system allows for batches of cartons to be pre-printed – particularly useful for small batch runs or where product is hand packed. Furthermore, with cartons being presented to the printer under ideal conditions a perfect print will be made each time.
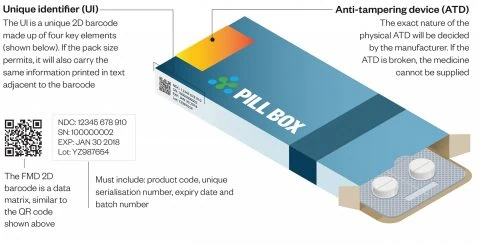
What is the FMD?
The Falsified Medicines Directive (FMD) aimed at reducing the number of counterfeit medicines infiltrating the legal pharmaceutical supply chain within Europe, was transposed into UK law in 2013.
Once implemented, the regulation will require individual packs to be serialised via unique codes encrypted in machine readable 2D datamatrix codes. Manufacturers who fail to meet this deadline will have to withdraw their products from the European market.
The challenge manufacturers face
Pharmaceutical manufacturers are all too aware of the need to comply with FMD; the challenge they face is how. The complexity of some of solutions is daunting not just from an integration and operational point of view, but also because of the cost. Our aim, in engineering this system was to make it much simpler. The RF Compliance is an easy-to-use, stand-alone system that doesn’t interfere with production or compromise line efficiency, says Richard Pether, Director at Rotech.
The RF Compliance succeeds Rotech’s RF1 Pharma, combining the modularity and automatic stack-to-stack feeding technology of the first generation coder with a state-of-the-art chassis and advanced controls that enable integrated printing, serialisation and inspection.
The system generates a unique code for each pack and transmits the codes to the printer on a carton-by-carton basis. Immediately after printing, an integrated camera inspects the barcode, OCR text and pharmacode. Linking all of these processes allows for maximum efficiency and ensures each pack is fully compliant.