New regulations, new packaging – what has changed?
As part of the Tobacco and Related Products Regulations (TRPR) 2016, there is now a requirement that all nicotine containing e-liquids and hardware which can hold such e-liquids i.e. Tanks, either manufactured or imported into the UK after the 19th November, must comply with strict labelling regimes. Although such products manufactured or imported prior to this date can continue to be sold, by the 19th May 2017 all such items must legally be in compliant packaging. So, what does this mean in reality and what should we expect to see when we purchase such items, either on-line or from a store in future?
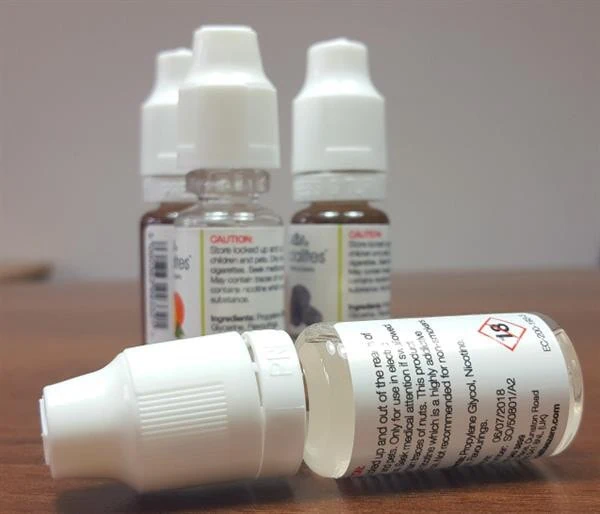
Labelling Requirements – E-liquid
- Health Warning – ‘This product contains nicotine which is a highly addictive substance’ must be present covering at least 30% of the 2 largest surfaces of the unit package.
- Ingredients must be declared on the packaging in descending order of weight, with the nicotine content clearly visible.
- Indication of the nicotine delivery per dose which the e-liquid will provide to the user.
- Details on the flavourings contained in the e-liquids, as this is not always obvious from the flavour name.
- Directions for use, including advise on the nozzle width of the bottle to allow consideration for the device it is suitable for filling, and also disposal of the contents and container.
- Batch coding must be present with details of the manufacturer/ importer for traceability.
- Storage instructions and shelf life of the product.
- Other relevant information relating to who the product is suitable for and any known side effects.
Other e-liquid regulations
The fact that an e-liquid is purchased and received in packaging including all of the above provides a clear visible indicator that the manufacturer/ importer has taken some action to comply with the requirements of TRPR, however there are several other key requirements which contribute to a product which is fully compliant and need to be considered such as bottle size and type, maximum strength, tests, notification to the MHRA etc.
Need help to ensure your products meet these requirements? Contact us.

